Intravenous catheterization is a common practice for administering fluids directly into the blood stream. It is often associated with a lethal and a very frequent complication of Catheter-related bloodstream infection (CRBSI).
Catheter-related bloodstream infections (CRBSI) are defined as the presence of bacteraemia originating from an intravenous catheter. CRBSIs are a leading cause of nosocomial infections associated with morbidity, mortality, and cost.
Managing CRBSI:
The general way of managing CRBSI associated with any local or systemic inflammation is to remove the catheters from patients and initiate antibiotic therapy empirically. Chlorhexidine gluconate impregnated transparent dressings are being used to cover and protect catheter sites and secure devices to the skin.
Do You Know?
Chlorhexidine belongs to the group of antiseptic antibacterial agents. It is primarily used to cleanse the skin after an injury, before surgery, or before an injection. Chlorhexidine works by killing (bactericidal) or preventing the growth of bacteria (bacteriostatic) on the skin. It can even be used to clean the hands before a procedure.
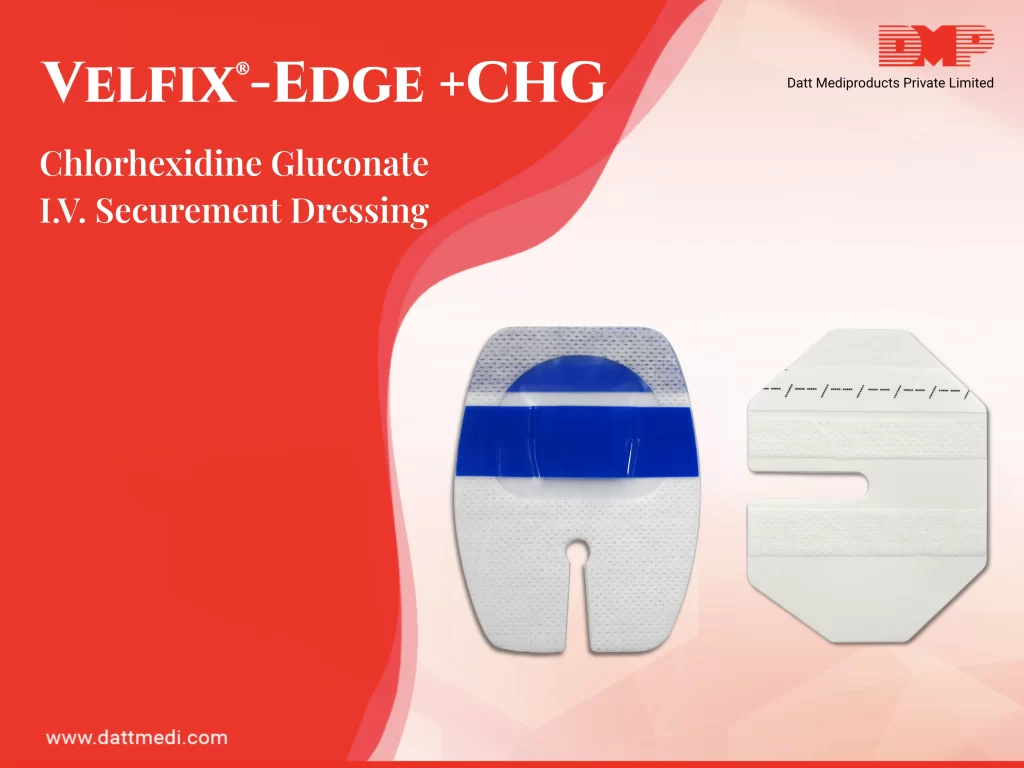
We present Velfix® -Edge+CHG, a Chlorhexidine Gluconate IV Securement Dressing. It comprises of a Stabilization Non-Woven Border, Antimicrobial Protection, High MVTR Transparent Film, a Notch, Securement Tape Strips and Documentation Label.
- Antimicrobial Protection offered by Chlorhexidine Gluconate which is effective in protecting against catheter tip colonization. As per the study published in The American Journal of Medicine, “Chlorhexidine has a broad spectrum of antibacterial activity against Gram-positive bacteria, Gram-negative bacteria, aerobic bacteria, anaerobic bacteria and fungi, and the use of chlorhexidine for skin disinfection in ICU patients reduces the spread of microbes and the incidence of CRBSIs”.
- Stabilization Non-Woven Border maximizes securement, breathability & wear time of the dressing.
- High MVTR Transparent Film acts as a waterproof sterile barrier to external contaminants. MVTR is Moisture Vapour Transmission Rate is the measure of permeability for vapour barriers. Velfix® -Edge+CHG is a high MVTR dressing which allows excess moisture to evaporate through the dressing while maintaining proper securement.
- Velfix® -Edge+CHG comes with 2 Securement Tape Strips to enhance the securement while promoting consistent application.
- A Notch is provided for opening to host the cannula’s port & reduces risk of catheter dislodgement.
- Documentation Label is a Pre-printed label to note dressing changes.
PROPERTIES & BENEFITS:
- Highly conformable to provide solutions for difficult catheter and IV sites
- Window frame design of Velfix® -Edge+CHG allows continuous observation of IV site
- Offers waterproof sterile barrier to external contaminants because of high MVTR transparent film
- Stabilization non-woven borders maximize securement, breathability & wear time
- Notched design discourages edge lift
- Velfix® -Edge+CHG is very easy to handle with gloved hands allowing single handed application
- Made with a premium 30 μ high MVTR transparent Polyurethane film
- EO Sterile
- Chlorhexidine gluconate (CHG) aids in infection reduction.
- Conforming & durable
- Leaves no adhesive residue on skin post removal
USE: Velfix® -Edge+CHG can be used for the securement of Central Venous Catheters, IV Catheter sites, Dialysis catheters, Hickman/ Broviac catheters, Epidural catheters, Arterial catheters, Short peripheral and midline venous catheters. Velfix® -Edge+CHG comes in various customized sizes with varying CHG gel pad sizes for different IV devices.




