Patent No.: US 10,293,075 B2
Date of the Patent Granted: May 21, 2019
Title of the Patent: Ready-to-use, Hydrophilic, Self-Dispersive, Fragmentable, and Biodegradable Porous Sponge Matrix and a Method of Manufacturing Thereof
NEED OF THE INVENTION:
Packing nasal and sinus cavities post functional endoscopic sinus surgery (FESS) is a routine procedure for most ENT surgeons. The packing is done to control postoperative haemorrhage and also to prevent the aspiration of blood in the postoperative period.
The packing also provides compression at the application site on the mucosal surface to inhibit tissue adhesion. These packs are made up of a variety of materials like cotton ribbon gauze, polyvinyl alcohol (PVA) sponge, Silastic balloon packs and are non-absorbable in nature.
Some centers even follow the traditional method of anterior nasal packing. This includes using cotton ribbon gauze saturated with liquid paraffin. Antibiotic ointments like Neosporin or bismuth iodoform paraffin paste (BIPP) may also be utilized.
These types of traditional nasal packings are often associated with significant patient discomfort and mucosal trauma and are preferably avoided, specifically after FESS, since these result in pressure necrosis, epistaxis upon removal, paraffin granulomas, and discomfort during placement and removal.
These dressings need to be removed and their removal is associated with various issues like pain, bleeding and makes the patient anxious. As per a study published in the Otolaryngol Head Neck Surg. Journal, patients consider the removal of a nasal pack as the most unpleasant part of the perioperative experience. Another study published in the American Journal of Rhinology shows that removal of nasal packs may cause mucosal trauma, which may lead to delayed healing and increased risk of scarring and synechiae formation.
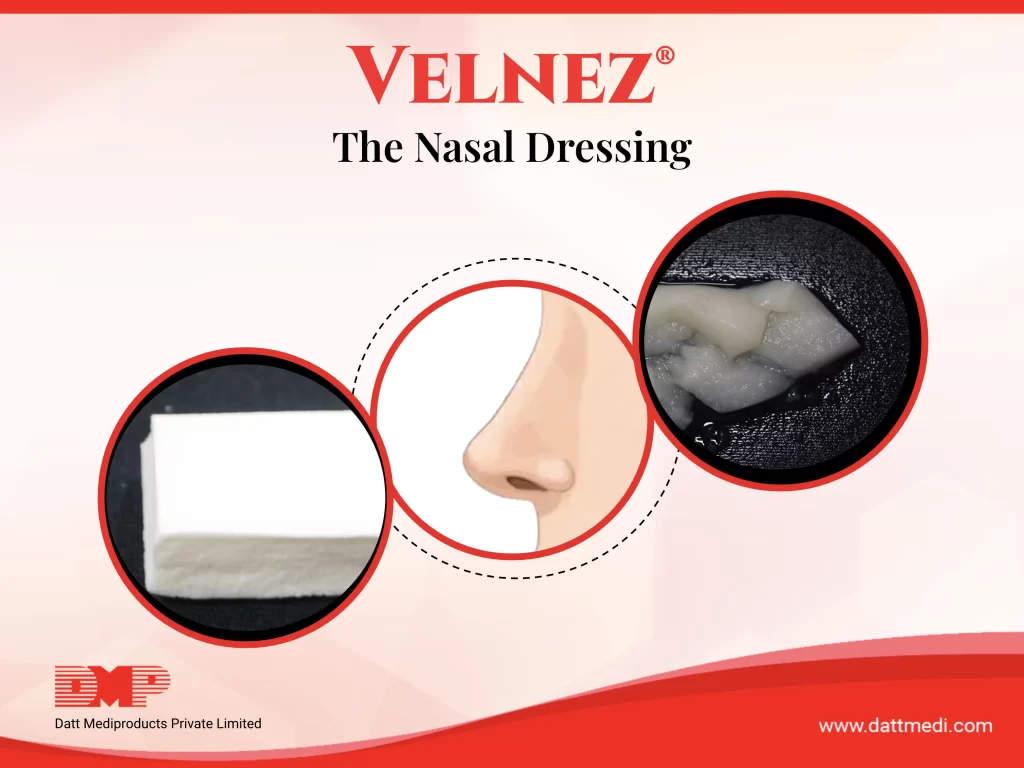
We at Datt Mediproducts Pvt Ltd are the only Indian company that manufactures and sell a biodegradable nasal pack. VELNEZ®, the present invention is now a patented product with a lot of patient and surgeon benefits.
ABOUT THE INVENTION:
- The invention (VelNez®) is a ready to use, self-dispersive, biodegradable and biocompatible device suitable for packing nasal and sinus cavities post surgeries.
- VelNez® acts as a space-occupying dressing and prevents adhesion by separating the compromised mucosal surfaces. It also reduces fibrosis at the same time and promotes healing and clotting.
- The porous scaffold of the invention also acts as a therapeutic carrier for drug delivery or to carry any bioactive molecules, anti-microbial agents or other chemicals.
- The porous sponge matrix comprises of a biocompatible and biodegradable polymer scaffold made up of a blend of synthetic and natural biopolymers as well as a 3-D scaffold polyelectrolyte complex (PEC) having vesicular micro-voids and an inner surface area larger than its outer surface area.
ADVANTAGES:
- The unique composition results in rapid and uniform fragmentation.
- The highly porous structure provides high absorption capacity.
- Supports tissue regeneration and wound healing process.
- Reduces fibrosis.
- Provides compression at the tissue site and maintains sufficient compression strength while absorbing the natural fluids present in the nasal cavity.
- Soft & flexible and can be easily cut and manipulated for surgeon preference and patient anatomy.
- No post-operative adhesions as it separates the compromised mucosal surfaces.
- No need for post-operative removal and hence atraumatic.
- Ergonomic design for patient comfort.
VelNez® fragments within a few days of application. The pain associated with the traditional nasal plug removal is completely eliminated with VelNez® since such procedure is not necessary on application of VelNez®.
Owing to these benefits, doctors are considering biodegradable dressings more than the counterparts these days. For more information on the nasal dressing and the differences in degradable and non-biodegradable nasal dressings, you can follow our previous blog.
You can also reach us by sending an email to support@dattmedi.com or visit our website www.dattmedi.com